

Research - (2022) Volume 8, Issue 9
Received: Sep 01, 2022, Manuscript No. JAEFR-22-73474; Editor assigned: Sep 05, 2022, Pre QC No. JAEFR-22-73474 (PQ); Reviewed: Sep 19, 2022, QC No. JAEFR-22-73474; Revised: Sep 26, 2022, Manuscript No. JAEFR-22-73474 (Q); Published: Oct 03, 2022, DOI: 10.3153/JAEFR.22.8.002
Citation: Shadrack Kwadwo Amponsah. Performance Assessment of Recirculating Aquaculture System (RAS) Designs for Culture of African Catfish (Clarias Gariepinus). J Aquacult Eng Fish Res 2022; 8(9).
This study comparatively assessed the technical and economic performance of two RAS designs for the culture of African Catfish (Clarias gariepinus). A total of 1000 Catfish fingerlings each were stocked in circular tarpaulin lined ponds installed with respective RAS designs (old and new). Data on fish growth and pond water quality were collected on bi-weekly and weekly basis, respectively. Pond water pH, temperature, Total Dissolved Solids (TDS) and Dissolved Oxygen (DO) were 6.30° C-6.60° C, 30.0° C-30.1° C, 144.1 mg/L-192.4 mg/L and 6.23 mg/L-6.47 mg/L for old and new RAS design, respectively. The new RAS design offered significantly favorable conditions than the old design, though both were within acceptable limits. Fish weight and length gain generally increased with increasing growth period (0-20 weeks) ranging from 4.85 g-687.45 g and 1.72 cm-35.21 cm, and 8.17 g-770.38 g and 6.04 cm-39.64 cm for old and new RAS designs, respectively. Significantly higher survival rate was recorded for new RAS design compared to the old. No significant difference was observed for Feed Conversion Ratio (FCR) and Specific Growth Rate (SGR) which ranged from 1.39 g/d to 1.39 g/d and 1.26 g/d to 1.30 g/d for old and new RAS designs, respectively. The newly designed RAS setup is more productive for catfish culture from an economic perspective with BCR of 1.20. Promotion and proper adoption of such RAS aquaculture technologies to boost urban and rural fish production could significantly impact the aquaculture sector
Aquaculture, recirculating, RAS design, Catfish, Dissolved oxygen
Aquaculture is a burgeoning sector that aims to meet the need for eating fish [1]. It involves rearing, breeding and harvesting of animals and plants in all types of water environments including ponds, rivers, lakes and the oceans [2]. Aquaculture has become one of the world’s fastest growing food producing areas. The United Nation’s Food and Agriculture Organization (FAO) agreed that worldwide fishery output needed increase by at least 50% to counter forecasted dietary protein shortages by 2030. The global wild fish harvest has remained stable at roughly 90 million tons per year, with little signs of increasing. However, demand for fish continues to rise, resulting in a massive expansion of the global aquaculture business. Currently, traditional fishing and aquaculture generate also contribute 1.5% to GDP and account for 7% of agricultural GDP [3].
According to Ahmad et al., a critical focus of the aquaculture sector globally is on the development and refinement of water recycling technology due to concerns about the negative effects of aquaculture production on the environment, greater regulations on aquaculture effluents and the need to save water resources and energy [4]. The dawn of the Recirculating Aquaculture System (RAS), according to Murray et al., commenced in the early 1950’s when earliest scientific researchers in Japan were interested in using water recirculation for aquaculture due to the shortage of flowing water for raising fish [5]. Recirculation Aquaculture System (RAS) is gaining popularity around the world, particularly in intensive finfish farming. This is owing to the perception that RAS significantly reduces land and water requirements while still providing a high level of performance. A level of control over the fish culture environment that allows for optimal development all year [6]. Low land requirements, low water requirements, the capacity to manage water quality, the ability to adjust water temperature, and independence from bad weather conditions are all advantages of recirculating aquaculture systems [7].
Recirculating aquaculture systems are being used in the production units of many tons of fish and aquatic creatures per year for consumption in several sections of the fish farming industry [8]. In terms of culture, Ghana uses dugout ponds, pens, cages, or raceways systems. Cages and raceways are expensive to operate in terms of structure, and feeding expenditures are substantially higher [9]. The farm operation is more dependent on the weather and other natural variables that are beyond the farmer's control in the dugout system. Amenyogbe et al., further explained that in these culture systems, water contamination occurs more frequently and drastic [9]. The RAS is a self-purifying aquaculture system that uses filters to treat contaminated water, eliminate solid wastes, and reduces the amount of water needed, as well as discharges from agricultural operations [10]. Despite the importance of RAS in aquaculture, the system is not well-known because there is little or no information available, especially in the case of developing countries. Unlike developed countries where extensive research and performance characterization has been conducted on the RAS for several decades this technology remains a grey research area for most developing countries [6,8,10-13]. Research and promotion of the RAS for Tilapia and African Catfish production in Ghana has been ongoing for almost a decade now, all geared towards an all-inclusive improved inland fish production. Amponsah reiterated this by stating that technological advancement through research has brought a new system of fish culture in simple raised tanks that has made it possible to rear fish even in the backyard with minimal skill requirement [14]. Due to the different types of filtration systems that have been created for RAS, all to manage and decompose ammonia and nitrite, and collect solid waste, there is also great debate and competition as to the most appropriate filtering system fit for the RAS set up. This research will add to our understanding of the RAS and the effects of biological filtration on fish production and the overall technical and economic performance.
Study objectives
The main aim of this research was to evaluate the performance of two RAS designs for cultivation of African Catfish (Clarias gariepinus).
Specifically, the study sought to:
• Assess the effect of the RAS design on water quality in ponds under Catfish production.
• Assess the influence of RAS design on the growth and development of Catfish.
• Assess the economic feasibility of using the RAS setup for Catfish production.
Study area
The study was conducted at the Cottage on the premises of CSIR-Crops Research Institute, Fumesua near Kumasi, GHANA.
RAS setup and operation principle
Circular collapsible tanks (made of canvas lining material) of approximately 13 m3 volume each were used for the experiment. Amponsah et al., reports that circular tanks facilitate waste removal and promote better pond water quality management [15]. A RAS was installed on the tank to complete the setup. The RAS works by first eliminating biosolids through mechanical filtration and then breaks down harmful matter in filtered water via a biological filtration process. The biological filtration (biofilter) setup is the single most important component of the pond system as far as waste management is concerned [15]. Bio filters use natural processes and bacteria through the nitrogen cycle to break down ammonia into less harmful components, a standard bio-filter is composed of submersible pumps, water hoses, nylon mesh stuffed bucket on a stand, nylon meshed stuffed basket inside the waste tank, sedimentation tank with its accompanied plumbing components [16].
For the typical RAS setup (control) installed for this experiment, a submersible water pump (6000 L/h) placed at one end of the pond periphery directs water flow in centrifugal motion to get biosolids to settle at the center of the tank. A series of water hoses (¾ inch diameter) transport (by gravity) biosolids into a sedimentation tank where mechanical filtration (using untwisted nylon mesh) takes place (Figure 1).
A second submersible water pump (1500 L/h) then conveys the filtered water through a biofilter, where nitrite in Ammonia (NH3) is broken down into nitrate before returning to the tank and the cycle continues. The distinguishing factor between the old RAS design (Figure 1a) and the new design (Figure 1b) is the substitution of the flexible water hose with PVC pipe suction mat to remove biosolids.
Experimental setup
Three tanks per RAS arrangement were installed. Each tank had 1000 African Catfish (Clarias gariepinus) fingerlings weighing 12.82 g ± 0.4 g. Before stocking, tanks were cleaned and sterilized with brine solution to remove hazardous microbes. The study used pelleted fish feed (Ranaan) of various sizes. The fish were fed thrice daily based on percentage body weight between 8.00 h-9.00 h, 14.00 h-15:00 h, and 20:00 h-21:00 h. The feed ration was periodically modified when necessary. Fish faeces and biosolids were disposed daily through the biofilter setup. Water from a mechanized borehole was used for the experiment and tanks were topped up twice weekly. The tanks were exposed to the sun (uncovered) and checked daily for dead fish and system performance.
Experimental design and data collection
Fish growth features were recorded bi-weekly on 10 fishes sampled randomly from each tank using a scoop net. Live fish were returned to their respective tanks after measurement. A Randomized Complete Block Design (RCBD) of 3 replicates was adopted for this experiment. RAS design was the single factor, growth period was blocked, and the responses were the measured fish growth characteristics (length increment, weight gain, specific growth rate, feed conversion ratio and mortality). The weight (grams) of individual fish was determined with an electronic sensitive scale (FF 1976 electronic weighing scale). Total length measurements (centimeters) were determined with a measuring rule. Growth parameters were calculated following the method of Bagenal and Gerking in equations 1-5 [17]:
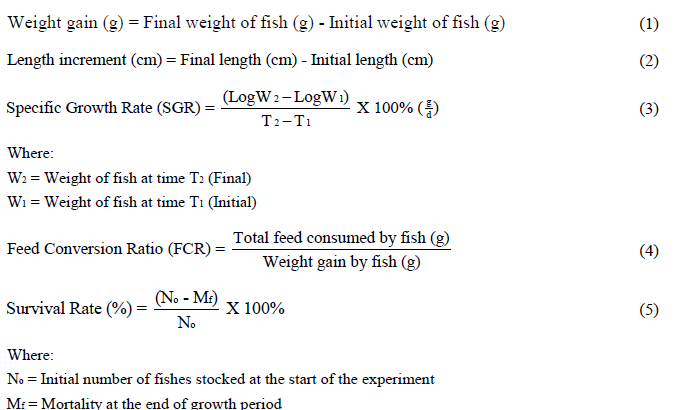
Water quality assessment
Water quality assessment for each tank was carried out every 7 days before 9 hours. Parameters monitored included dissolved oxygen (DO), pH, water temperature and total dissolved solids (TDS) using the 86031 AZ waterproof IP67 Combo water quality tester. 3 water samples each were collected from the pond, mechanical filter bucket and the biological filter setup and tested to allow for comparison.
Statistical analysis
Analysis of variance (ANOVA) on data set collected was done using GenStat statistical package version 11 [18]. Least Significance Difference (LSD) among treatment means was determined at P ≤ 5% significance level.
Economic assessment
Benefit Cost Ratio (BCR) was determined for Catfish production in tanks installed with the respective RAS designs to measure the cost effectiveness of each production system. BCR was calculated using Equation 6 following the method of Shively G [19]:

Where: Bt = Total benefit at time, t
Ct = The measure of costs at time, t
Water quality
From Table 1, pond water pH, temperature, Total Dissolved Solids (TDS) and Dissolved Oxygen (DO) ranged from 6.30°C to 6.60°C, 30.0°C to 30.1°C, 144.1 mg/L to 192.4 mg/L and 6.23 mg/L to 6.47 mg/L for old RAS design (RD1) and new RAS design (RD2), respectively. In comparison to optimal water quality standards, pond water pH and total dissolved solids (TDS) for RD2 were significantly desirable than for RD1 (Table 1) [20-23].
The relationship observed between pH and TDS (Table 1) agrees with study by Faysal M et al., and affirms the assertion that the higher the TDS, the lower the pH, towards acidity [24]. Water temperature and dissolved oxygen for both RAS designs were not significantly different though they fall within acceptable limits. Chimits P also cited that Clarias gariepinus sp. could tolerate temperature variations between 20°C-35°C [25].
Irrespective of the RAS design, pH and DO values at the biofilter, mechanical filter and tank collection points showed significant difference. Water in the tank recorded the highest pH and DO as compared to other data points. The least pH of 6.32, which is below the optimum range, was recorded in the mechanical filter. The mechanical filter is a point where raw faecal and feed waste are first collected from the pond and filtered. There is a possibility that some raw waste could escape unfiltered, a situation that may create undesirably high acidity in pond water. This occurrence is particularly true especially in the case of the old RAS design which experiences some backflow of raw waste due to the configuration and layout of its mechanical filter unit. Similarly, the DO in the mechanical filter unit slightly exceeded the minimum acceptable limit of 5.0 mg/l, perhaps due to limited exposure of filtered water to atmospheric oxygen. According to expectation, the DO in tank water was highest due to the presence of an aeration system which continuously pumps and circulates atmospheric oxygen through the pond. Moreover, aside the large pond surface area, the action of water dropping from the biological filter unit (Figure 1) provides extra atmospheric oxygen.
Fish growth performance
The length of Catfish for both designs showed a general increase along the growth period (0 week-20 weeks) ranging from 1.72 cm to 35.21 cm and 6.04 cm to 39.64 cm for RD1 and RD2, respectively. This trend of increasing Catfish length with increasing growth period concurs with similar study by Amponsah et al., [2]. From Figure 2, the length increment of Catfish cultured in tanks with new RAS (RD2) was significantly higher compared to Catfish in tanks with the old RAS (RD1). The differences in pond water quality (Table 1) for both RAS designs could have resulted in the differences in length increment over the growing period (Figure 2).
| Water Quality Parameter | ||||
|---|---|---|---|---|
| Data point | pH | Temp (°C) | TDS (mg/L) | DO (mg/L) |
| Biof | 6.467a | 29.87 | 152.6 | 6.0b |
| Mech | 6.316b | 30.08 | 182 | 5.2c |
| Tank | 6.493a | 30.22 | 170.2 | 7.86a |
| LSD | 0.0748 | ns | ns | 1.023 |
| Design | ||||
| RD1 | 6.295b | 30.02 | 192.4a | 6.47 |
| RD2 | 6.556a | 30.09 | 144.1b | 6.23 |
| LSD | 0.0611 | ns | 30.96 | ns |
| Optimal range | 6.5-9.01 | 22-352,1 | ≤ 10003 | ≥ 54 |
A general increase in mean weight gain of Catfish was observed for both RAS designs (RD1 and RD2) with increasing growth period (Figure 3). Weight gain ranged from 4.85 g to 687.45 g and 8.17 g to 770.38 g for RD1 and RD2, respectively along the production period (0 weeek-20 weeks). Catfish in RD2 improved significantly in weight compared to those cultured in RD1. This difference in weight gain could best be attributed to water quality since factors that affect growth are linked to environmental conditions (Figure 3) [26].
The average survival rate of Catfish in RD1 and RD2 were 93.28% and 95.03% respectively (Figure 4). A general perspective of the results showed higher survival rate of Catfish in tanks with RD2 compared to RD1. This could perhaps be attributed to the differences in culture conditions for both RAS designs since poor water quality may cause high mortality and vice-versa (Figure 4) [27].
From Table 2, feed conversion ratio (FCR) for Catfish ranged from 1.393 to 1.394 for RD2 and RD1, respectively, whereas specific growth rate (SGR) ranged from 1.26 g/d to 1.30 g/d for RD1 and RD2, respectively. It was observed that there was no significant difference in FCR and SGR for both RAS designs, irrespective of the growth period (Table 2).
|
|
Fish Growth Parameter |
||||
|---|---|---|---|---|---|
| RAS Design | Length increment (cm) | Weight gain (g) | Survival rate (%) | FCR | SGR (g/d) |
| RD1 | 22.59b | 233.6b | 93.28b | 1.394 | 1.256 |
| RD2 | 26.74a | 298.7a | 95.03a | 1.393 | 1.301 |
| LSD | 1.994 | 18.09 | 1.208 | ns | ns |
Cited low dissolved oxygen level as the major limiting water quality variable which has implications on fish survival and feed conversion efficiency [28].
Economics of catfish culture with RAS
The results of the economic analysis in Table 3 illustrates that the benefit cost ratio (BCR) for the old and new RAS designs were 1.02 and 1.20, respectively. Clearly, the result indicates that the old RAS design offers higher economic returns than the old design for culture of African Catfish (Clarias gariepinus) (Table 3).
| Cost Item | Unit | Quantity | RAS Design | |
|---|---|---|---|---|
| RD1 | RD2 | |||
| Pond setup (tank+RAS) | GHâ?µ | 1 pc | 7300 | 7300 |
| Fingerlings (@GHâ?µ1.50) | GHâ?µ | 1000 pcs | 1500 | 1500 |
| Fish feed (@GHâ?µ 155) | GHâ?µ | 30 bags | 4650 | 4650 |
| Total fixed and variable cost | GHâ?µ | 13450 | 13450 | |
| Survival rate | % | 93 | 95 | |
| Quantity of fish at end of cycle | pcs | 930 | 950 | |
| Av. fish weight (@20 weeks) | kg | 0.74 | 0.85 | |
| Cost per kg of fish | GHâ?µ | 20 | 20 | |
| Total revenue (fish sales) | GHâ?µ | 13764 | 16150 | |
| Total profit at end of production | GHâ?µ | 314 | 2700 | |
| Benefit Cost Ratio (BCR) | 1.02 | 1.2 | ||
The new RAS design offers more favorable water quality conditions over the old design, especially for critical parameters such as pH and Total Dissolved Solids (TDS). Generally, water quality parameters for both RAS designs were within acceptable limits. Growth assessment of Catfish under the new RAS design was significantly better than the old design, especially for length increment, weight gain and survival rate. The newly designed RAS setup is more productive for catfish culture from an economic perspective. The need to promote the new RAS design for culture of Catfish is therefore justified.
Further study should consider a comparative assessment of tank culture of Catfish and Tilapia with and without the new RAS design. Promotion and proper adoption of such RAS aquaculture technologies could boost inland fish production and substantially impact the aquaculture sector.
None.
The author declares there is no conflict of interest in publishing this article.
[Crossref]